Meeting environmental, health, safety, and quality (EHSQ) standards in complex industrial environments can feel overwhelming. Non-conformities have far-reaching consequences from putting safety at risk and causing environmental damage to attracting regulatory penalties and damaging your organization’s reputation…the list goes on.
But fear not, there’s a solution that can help you tackle non-conformities head-on. It’s time to implement CAPA Management (Corrective Action and Preventative Action) software.
In this blog post, we’ll address the common challenges faced during the CAPA process and uncover the key advantages of utilizing software to streamline your CAPA management.
Are you ready to take your EHSQ program from a state of chaos to one of control and efficiency? Let’s dive in!
Why Does CAPA Matter?
CAPA Management provides a systematic and proactive approach to identify, investigate, and eliminate problems that may arise in your processes, products, or services. It’s an indispensable component of safety and industrial standards worldwide, empowering you to mitigate risks and ensure compliance with regulatory requirements.
With CAPA Management, you gain the power to address issues before they arise, fostering a culture of continuous improvement and ensuring the highest standards of quality and safety in your operations.
What Common CAPA Management Challenges Can You Face?
Manual CAPA management processes are labor-intensive. Every step, from filling out forms to maintaining spreadsheets and sending countless emails across departments, is time-consuming. Add that’s before the hours wasted sifting through decentralized and inconsistent data.
When various departments rely on multiple spreadsheets and physical files, miscommunication, information loss, and delays are all part of the package. Inconsistencies and data entry errors become the norm, leading to incorrect analysis and poor decision-making.
The lack of real-time visibility in manual systems makes it nearly impossible to get an immediate view of non-conformity status or CAPA activities, causing frustrating delays in resolving critical issues. And, spotting trends or identifying root causes feels like searching for a needle in a haystack. This results in repeated mistakes and missed opportunities for improvement.
Without software, keeping up with regulatory changes and producing necessary documentation during audits often feels like an uphill battle. Compliance risks become a constant headache.
And if all that wasn’t enough, follow-ups to verify the effectiveness of corrective or preventive actions tend to fall through the cracks in manual systems, reducing the overall impact of the CAPA process.
How To Take Back Control of Your CAPA Management
When it comes to managing non-conformities and CAPA, implementing specialized EHSQ software, just like that provided by TenForce, can be a game-changer. Here’s how it can support your efforts and make your life easier:
- Automate your entire EHSQ workflow, from identifying non-conformities to taking corrective and preventive actions. It saves you time and resources by initiating actions promptly and tracking them systematically.
- Stop human errors from messing up your data. Maintain consistent metrics and terminologies to improve data accuracy and make better decisions based on reliable information.
- Say hello to easy access and efficient organization. Access and manage your CAPA-related data in one central platform, from incident reports and documentation to corrective actions and preventative actions.
- Gain instant insights and real-time visibility through customizable dashboards and detailed reports. Ensure complete traceability of non-conformities from detection to resolution.
- Keep everyone on the same page with better communication and collaboration between teams. And don’t worry about missing important information. The alert and notification system has got your back.
- Unleash the power of analytics to identify patterns and trends in non-conformities. By understanding the root causes, you can supercharge your CAPA efforts, track key performance indicators, and drive continuous improvement in your EHSQ processes.
- Keep up to date with regulatory changes and align your processes accordingly. Software simplifies audits, manages necessary documentation, and minimizes compliance risks. Stay compliant and worry-free.
- Never miss a beat! Receive automatic reminders for pending actions. Automation reduces manual effort, ensures timely execution of CAPA plans, and maintains a thorough and effective CAPA process.
Conquering CAPA Management with TenForce
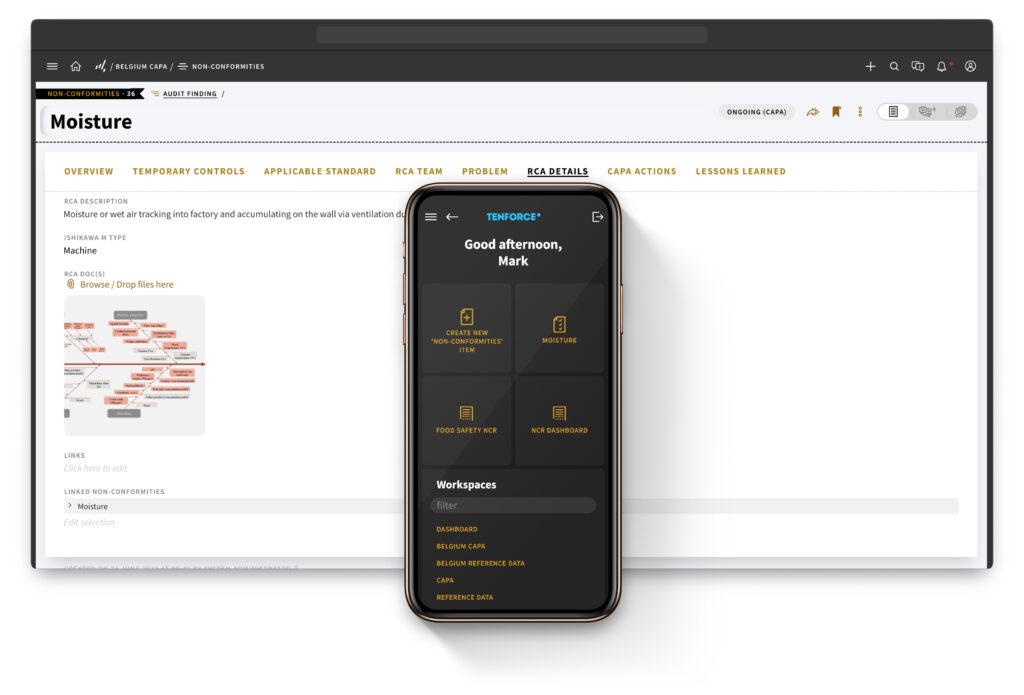
Now that you’re up to speed on the significance of CAPA management, the challenges you might face, and the advantages of software solutions, it’s time to take the next step.
Watch our free on-demand webinar for a behind-the-scenes demo of TenForce CAPA Management in action. And, there’s more exciting stuff coming your way! Stay tuned for our comprehensive guide to CAPA management, coming soon.
Get ready to seize control and embark on the journey to operational excellence with TenForce.
